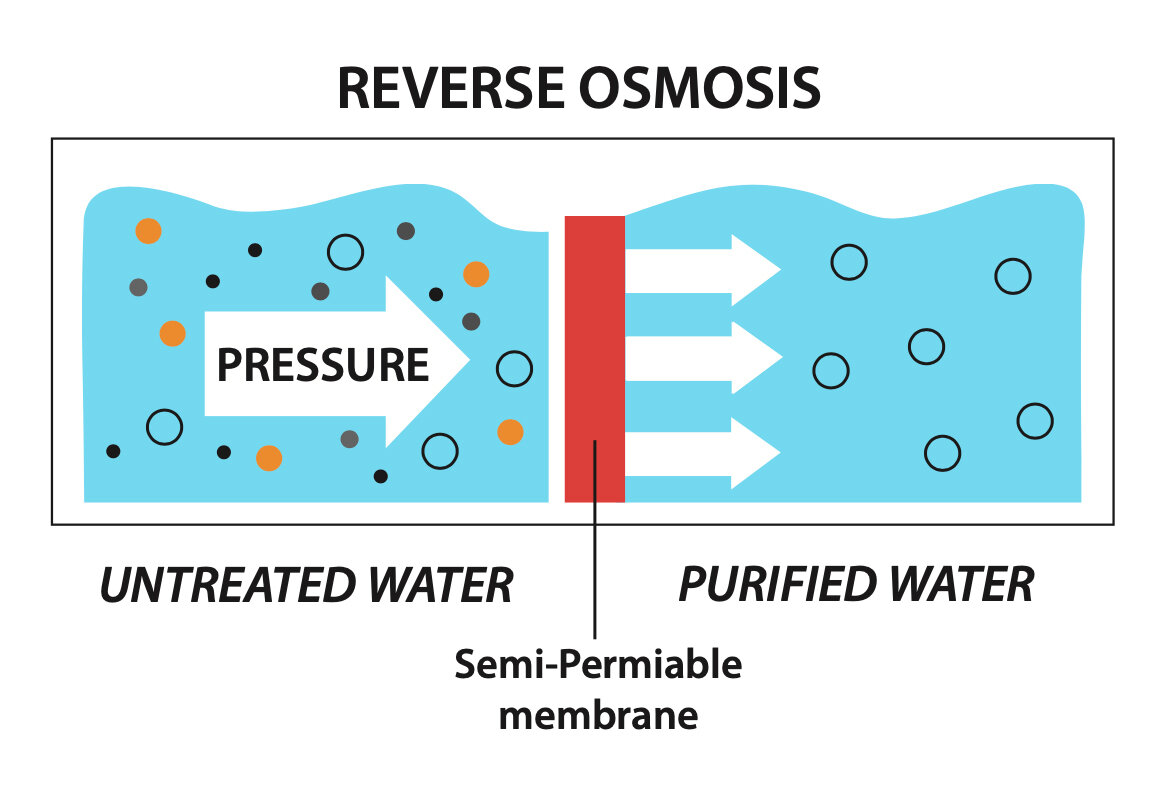
How Does Reverse Osmosis Work?
Public water suppliers work hard to provide clean water for their customers. The problem is that there are many contaminants, especially those that cause taste and odor issues, which are not EPA regulated. These contaminants can easily penetrate aquifers, streams, and rivers, bringing impurities straight to your water lines. That’s where Reverse Osmosis comes in. Reverse Osmosis safely removes Fluoride, Lead, Chlorine and Chloramine, Pesticides, Detergents, and more.
With a Reverse Osmosis filtration system, you can filter out impurities and produce outstanding drinking water for your home or business. Reverse Osmosis (RO) is a purification process that is simple and straightforward. Water pressure pushes tap water through a semipermeable membrane to remove impurities from water. During this process, dissolved inorganic solids (such as salts) are removed from drinking water.
The membranes used in RO are generally composed of a non-porous polymeric film underlain by porous support layers. The openings in this polymeric film are very small, which allows RO the unique ability to remove most dissolved solids from water. Specifically, RO is capable of rejecting viruses, bacteria, salts, sugars, proteins, particles, dyes, heavy metals, dissolved organics, and other contaminants.
Total Dissolved Solids (TDS) is a measure of the combined content of all inorganic and organic chemicals dissolved in water. Examples of inorganic chemicals that commonly contribute to a measurement of TDS include calcium, magnesium, potassium, sodium, bicarbonate, chloride, and sulfate. Organic chemicals that may contribute to TDS can derive from land application of chemicals, the industrial release of chemicals to the environment, vegetable matter, and animal matter. In practice, handheld meters use a conductivity measurement to approximate the TDS in water.